These tables list values of molar ionization energies measured in kJmol 1This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. C 10 H 8.

Molar Mass How To Find Molar Mass Molar Mass Of Water Molar Mass Atomic Mass Unit Chemistry
Molar concentration is the amount of a solute present in one unit of a solution.

. We can also use molecular weight calculator for finding molar mass of a. This way we can calculate the molar mass of a compound or one-carbon compound. Calculate the enthalpy change for the reaction.
While adding up the atomic masses to find molecular mass do not forget to consider the number of atoms of each element. For example the Chlorine Cl has a molar mass of 354530 gmol in the same way Sodium Na has a molar mass of 229898 gmol. The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit amu.
Calculating the molar enthalpy of neutralisation from experimental results is a 3 step process. 5 then molar mass ratio of these isotopes is x. Thus we put the values in the formula to find atomic mass such as.
When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. To prepare 1 L of 05 M sodium chloride solution then as per the formula use 2922 g of sodium chloride. However some of these ions associate with each other in the solution leading to a decrease in the total number of particles in the solution.
Some facts The molar mass is simply the mass of one mole of substance ie. If the formula used in calculating molar mass is the. 4 7 C l 3 7 which has a mass of 3 6.
In other words set the mass of each element equal to the percent. If we have to measure one mole of sodium. Well add those numbers together along with the unit grams per mole for finding molar mass.
Thus by knowing the molar mass we can determine the number of moles contained in a given mass of a sample. Would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams. A periodic table is necessary to complete the questions.
The first molar ionization energy applies to the neutral atoms. If you have a subscript in a chemical formula. Atomic mass of Carbon 1201.
Definitions of molecular mass molecular weight molar mass and molar weight. Moreover most of the molecules are made up of more than 1 element. To calculate the molecular mass sum up the atomic masses of all the elements present in the compound.
The second third etc molar ionization energy applies to the further removal of an electron from a singly doubly etc charged ion. Use this periodic table for calculating molar mass for any chemical formula. Let me make it more clear with an example of sodium chloride.
Examples of molecular weight computations. Molar mass g mol octadecane. Molar mass of Carbon Monoxide 2801.
It is 5844 g mol 1. Its units are molL moldm 3 or molm 3. Use the molar mass you get by adding up the atomic weight of the elements from the periodic table to convert the mass of each element into moles.
9 6 6 a m u. Finding molar mass starts with units of grams per mole gmol. IOS app is also available.
This collection of ten chemistry test questions deals with calculating and using molar masses. 1 where x is. Atomic mass of Oxygen 1600.
Calculate the heat evolved. View solution Naturally occuring chlorine is 7 5. One molecule of water H 2 O would weigh 1802 amu 2100797 amu for H 159994 amu for O and a.
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. For example the Vant Hoff factor of CaCl 2 is ideally 3 since it dissociates into one Ca 2 ion and two Cl ions. 9 6 9 a m u and 2 4.
If we write this as a calculation it looks like this. Isocane eicosane C 20 H 42. Molar mass g mol chlorine.
The molar mass of a substance is the mass of one mole of the substance. Also the periodic table can help you to. Chloroacetic acid was first prepared in impure form by the French chemist Félix LeBlanc 18131886 in 1843 by chlorinating acetic acid in the presence of sunlight and in 1857 in pure form by the German chemist Reinhold Hoffmann 18311919 by refluxing glacial acetic acid in the presence of chlorine and sunlight and then by the French chemist Charles Adolphe.
1 atom x 23 gramsmole Na 1 atom x 355 gramsmole Cl 585 gramsmole NaCl. Divide each mole value by the small number of moles you obtained from your calculation. The answers appear after the final question.
Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter its formula specify its isotope mass number after each element in square brackets. If the atomic mass of chlorine is 3 5. The molar mass links the mass of a substance to its moles.
The total should be 100 percent. 60663 Calculate the. 5 3 C l 3 5 which has an atomic mass of 3 4.
This factor is named after the Dutch physical chemist Jacobus Henricus Vant Hoff who won the first Nobel Prize in chemistry. If we have a chemical compound like NaCl the molar mass will be equal to the molar mass of one atom of sodium plus the molar mass of one atom of chlorine. So for finding out the molar mass of the molecule you have to break it into elements to know its molar mass.
C 18 H 38. The molar mass of sodium chloride is known. Calculate the average atomic mass of chlorine.
A 9 8. Molar concentration also known as molarity and can be denoted by the unit M molar. 1 7 3 5 Cl and 1 7 3 7 Cl are two.
The mass of the sample containing about 6 023 1 0 23 6023 times 10. Q m C g T m total mass of reaction mixture C g specific heat capacity of solution ΔT change in temperature of solution Step 2. What is molar concentration.
Options for hiding the symbol or name of the elements provide a handy learning aid for memorizing the periodic table.
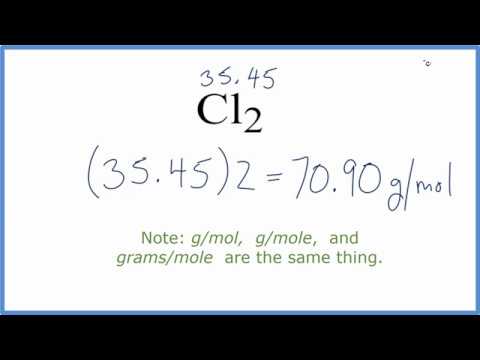
Molar Mass Molecular Weight Of Cl2 Chlorine Gas Youtube


0 Comments